 Jumat, 24 Januari 2025 (05:01)
Jumat, 24 Januari 2025 (05:01)
 Music |
 Video |
 Movies |
 Chart |
 Show |
 |
Solubility product of silver bromide is \( 5.0 \times 10^{-13} \). The quantity of potassium bro... (PW Solutions) View |
 |
Solubility product of silver bromide is \( 5.0 \times 10^{-13} \). ... (PW Solutions) View |
 |
Solubility product of silver bromide is `5.0xx10^(-13)`. The quantity of potassium bromide (Doubtnut) View |
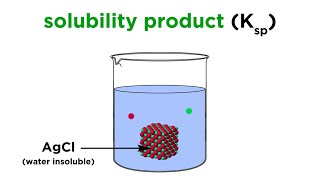 |
Solubility Product Constant (Ksp) (Professor Dave Explains) View |
 |
CH8-1 Problem Set: Question 6 (Clayton Spencer) View |
 |
Ksp calculation example (Debbie Leedy) View |
 |
15.54 | Calculate the molar solubility of AgBr in 0.035 M NaBr (Ksp = 5 × 10^–13) (The Glaser Tutoring Company) View |
 |
Chem 8.5 - Ksp Solubility Product (Sci Yeung) View |
 |
AIEEE Chemistry Paper 2010 AIEEE-2010, IONIC EQUILIBRIA, SOLUBILITY PRODUCT (Learners' Planet) View |
 |
Lec 3a. Common Ion Effect (jchemie) View |